In 1989, first CAR-T cells were developed by Gideon Gross and Zelig Eshhar at Weizmann Institute, Israel.
The complexity of the engineered CAR receptors has evolved over time, and depending on their composition, they are referred to as first, second , third, or fourth generation CARs.
Since the conventional cornerstones of cancer treatment (surgery, chemotherapy, and radiation therapy) manifest more and more limitations against multiple human malignancies, the novel immunotherapy is growing excitement with stutter steps, particularly the CAR-T therapy.
Hailstones as “a living drug”, CAR-T is an admirable example of the application of basic research to the clinic.
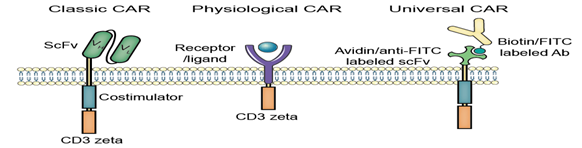
Representative CARs
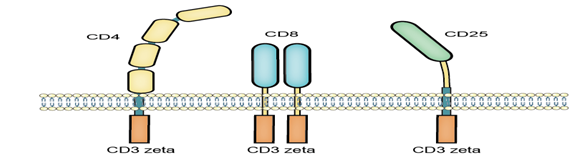
The first fusion receptors
CAR stands for chimeric antigen receptor or artificial T cell receptor, which is engineered abundantly and endowing an immune effector cell (T cell) with an uninformed specificity via a monoclonal antibody.
The original CAR-T or the first-generation CAR consists of the intracellular domain from the CD3 ζ- chain and the primary transmitter of signals from the endogenous TCRs, which showed success in pre-clinical trials and entered in Phase I clinical trials in ovarian cancer, neuroblastoma and various types of leukaemia and lymphoma.
Even though the anti-tumor activity was limited due to insufficient activation, determination and homing to the cancer tissue, some significant effects certainly existed in patients with B-cell lymphoma treated with α-CD20-CD3 ζ CAR-modified T cells and also some neuroblastoma treated with ScFv-CD3 ζ CAR-Ts.
This is the most common form of CARs in order to fuse single-chain variable fragments (scFv) derived from monoclonal antibodies to CD3 ζ transmembrane and endo domain.
To augment the antitumor efficiency of 1st-generation CARs, the 2nd-generation CARs were designed to combine the intracellular signaling domains from various co stimulatory protein receptors (e.g., CD28, 41BB, ICOS) that are incorporated in the cytoplasmic tail of the CAR to enhance the signaling.
For instance, the CD19-targeted CARs incorporated with CD28 or 4-1BB signaling domains manifested outstanding complete remission rates in patients with refractory B-cell malignancies.
consequently, the CD28-based CARs showed a brisk proliferative response and boost effector functions. Meanwhile, the 4-1BB-based CARs manifested a other progressive T cell accumulation.
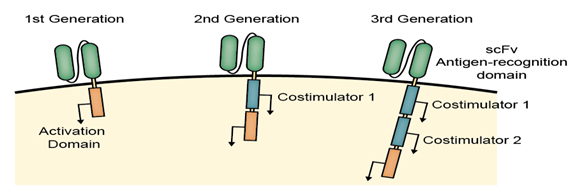
The three generations of CARs
As the expectation of more antitumor efficiency, the 3rd-generation of CARs combined with multiple signaling domains (e.g., CD3 ζ-CD28-41BB, CD3 ζ-CD28-OX40) to acquire additional enhanced activation signals, proliferation, production of cytokines and effective function.
For instance, the α-CD19-CD3 ζ-4-1BB CAR-Ts for chronic lymphocyte leukemia illustrates complete remission to infiltrate and lyse cancer tissue. Even better, a portion of CAR-Ts functioned as a memory phenotype for preventing the tumor relapses.
Despite the significant therapeutic effect, the emerging unmanageable activity accompanied with more antitumor efficacy caused life-threatening lysis activity as the most critical adverse effect or toxicity including clinically significant release of pro-inflammatory cytokines, pulmonary toxicity, multi-organ failure, and ultimate death.
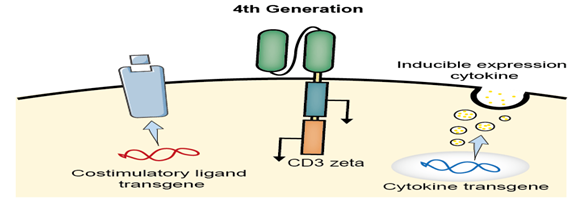
The 4th generation CAR
The previous CAR strategies are extremely specific and useful in redirecting T cells targeting malevolent cancer cells. However, the major limitation on solid tumors with a tremendous phenotypic heterogeneity and relapse due to antigen-negative cancer cells is the huge confront to trigger a novel CAR strategy.
The 4th-generation CAR-T is designed to shape the tumor environment by the inducible release of the transgenic immune modifiers, such as IL-12, which augments T-cell activation, attracts and activates native immune cells in order to eliminate antigen-negative cancer cells in the targeted lesion.
Control mechanisms: Adding a synthetic control mechanism to engineered T cells allows doctors to precisely control the persistence or activity of the T cells in the patient’s body, with the goal of reducing toxic side effects.
The major control techniques that trigger T cell death or limit T cell activation, and often regulate the T cells via a separate drug that can be introduced or withdrawn as needed.
Suicide genes: Heritably modified T cells are engineered in order to include one or more genes that can induce apoptosis when activated by an extracellular molecule.
Herpes simplex virus thymidine kinase (HSV-TK) and inducible caspase 9 (iCasp9) are two types of suicide genes that have been incorporated into CAR-T cells.
ON-switch: In this system, CAR-T cells can only function in the existence of both tumor antigen and a benign exogenous molecule. To achieve this, the CAR-T cell’s engineered chimeric antigen receptor is rip into two separate proteins that must come together to function.
The first receptor protein usually contains the extracellular antigen binding domain, while the second protein contains the downstream signaling elements and co-stimulatory molecules (such as CD3ζ and 4-1BB).
In the presence of an exogenous molecule (such as a rapamycin analog), the binding and signaling proteins dimerize collectively, allowing the CAR-T cells to attack the tumor.
Small molecule drug conjugates adaptor technology
SMDCs (small molecule drug conjugates) platform in immuno-oncology is an experimental approach that makes feasible the engineering of a single universal CAR T cell, which binds with extraordinarily high resemblance to a benign molecule designated as fluorescein isothiocyanate (FITC).
Also these cells are used to treat various cancer types when co-administered with bispecific SMDC adaptor molecules. These unique bispecific adaptors are constructed with a FITC molecule and a tumor-homing molecule to accurately bridge the universal CAR T cell with the cancer cells, which causes localized T cell activation.
Anti-tumor activity in mice is induced only when both the universal CAR T cells and the correct antigen-specific adaptor molecules are present. By adjusting the administered adaptor molecule dosing, anti-tumor activity and toxicity can be controlled.
Treatment of antigenically varied tumors can be achieved by administration of a mixture of the desired antigen-specific adaptors.
There are several challenges of current CAR T cell therapies, such as:
- the inability to control the rate of cytokine release and tumor lysis
- the absence of an off switch that would terminate cytotoxic activity when tumor abolition is complete
- a requirement to generate a different CAR T cell for each unique tumor antigen may be solved or mitigated using the adaptor approach. https://www.creative-biolabs.com/car-t/car-design-construction.htm https://en.wikipedia.org/wiki/Chimeric_antigen_receptor_T_cell


Excellent blog you have got here.. It’s difficult to find high-quality writing like yours nowadays.
I truly appreciate individuals like you! Take care!!