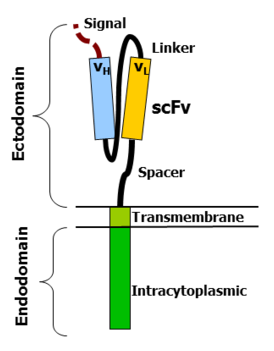
CAR-T Cell therapy is developed using the patient’s own T-Cells which are part of the body immune system and play a central role in fighting certain types of cancers . A patient’s T cells are extracted and reprogrammed outside of the body to recognize and fight cancer cells and other cells expressing a particular antigen.
The first two FDA-approved CAR-T therapies both target CD19 antigen and found on many types of B-cell cancers. Tisagenlecleucel (Kymriah) is approved in order to treat relapsed/refractory B-cell precursor acute lymphoblastic leukemia (ALL), while axicabtagene ciloleucel (Yescarta) is approved in order to treat relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
As of March 2019, there were approximately 364 ongoing clinical trials occurring globally involving CAR-T cells. The majority of those trials target blood cancers: CAR-T therapies account for above half of all trials for hematological malignancies. CD19 continues to be the most popular antigen target, followed by BCMA (generally expressed in multiple myeloma).
In 2016, studies began in order to explore the viability of other antigens, such as CD20.Trials for the solid tumors are less dominated by CAR-T, with about half of the cell therapy-based trials involving other platforms such as NK cells. Even though the initial clinical remission rates after CAR-T cell therapy in all the patients are as high as 90%, long term survival rates are much lower.
The cause is normally the emergence of leukemia cells that do not express CD19 and so avoid recognition by the CD19–CAR T cells, a phenomenon known as antigen escape.
Preclinical studies developing the CAR-T cells with double targeting of CD19 plus CD22 or CD19 plus CD20 have been demonstrated promise, and trials studying bispecific targeting to the circumvent CD19 down-regulation are ongoing.
How CAR-T Therapy Work 1.Leukapheresis A patients white cells,including T cells are extracted through a specialized blood filtration process known as Leukapheresis, then T -cells are cryopreserved and send to manufacturing unit for reprogramming.
2.Reprogrammed cells T cells are genetically encoded to recognize cancer cells by using viral vector (inactive virus)and other cells expressing a specific antigen.
3.Expansion Newly formed Car -T cells undergo expansion.
4.Quality checking Strict quality check occurs before release and shipment of the CAR-T cells back to patient.
5. Lymphodepleting Chemotherapy Lymphodepleting Chemotherapy is given to patient in order to reduce the level of WBCs and help the body accept the Reprogrammed cells
6 . Cell Infusion Reprogrammed CAR-T cells delivered into patient blood.
7. Cell Death Now CAR-T cell present in patient body have the potential to recognize the patient’s cancer cells and other cells expressing a specific antigen and attach to them,which may be start with direct cell death.
Kymriah (tisagenlecleucel, formerly CTL019) was the first CAR-T cell therapy approved by the US FDA. Kymria uses 4-1BB costimulatory domain, which is critical for full activation of the therapy, enhancement of the cellular expansion and durable persistence of the cancer-fighting cells.
The approved indications for the treatment of pediatric and young adult patients up to 25 years with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant or in the second or later relapse; and for the treatment of adult patients with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL) after two or more lines of the systemic therapy. Route of administration: intravenous infusion. Reasons to delay Kymriah treatment
Kymriah treatment should only be delayed, if the patient has any of the following conditions: Unresolved serious adverse reactions (particularly pulmonary reactions, cardiac reactions or hypotension) from the preceding chemotherapies. vigorous uncontrolled infection. Active graft-versus-host disease (GVHD). considerable clinical worsening of leukemia burden or lymphoma following lymphodepleting chemotherapy.
Limitation
of Use:
KYMRIAH is not indicated for the treatment of patients with primary central
nervous system lymphoma. Pregnancy, Lactation, Females and Males of Reproductive Potential(No data are accessible of
KYMRIAH use for the pregnant or
lactating women)
Adverse effects:
KYMRIAH have adverse effect such as Cytokine
release syndrome and neurological toxicities and allergic rections
.
- Cytokine Release Syndrome (CRS) includes fatal or
life-threatening reactions, occurs in
patients receiving KYMRIAH. Patients with active infection or inflammatory
disorders must not administer KYMRIAH.
Treat severe or life-threatening CRS with the tocilizumab, or tocilizumab and
corticosteroids.
- Neurological toxicities, which may be severe or
life-threatening and can occur following treatment with KYMRIAH.
- Allergic reactions may also
occur with KYMRIAH like serious hypersensitivity reactions, including
anaphylaxis, may be due to dimethyl sulfoxide or dextran 40 in KYMRIAH.
Monitoring
after Kymriah infusion: Kymriah may cause side effects that might be severe, life-threatening or
fatal. As a result, patients should be monitored daily for the first
10 days following the infusion for signs and symptoms of the potential
cytokine release syndrome, neurological events and other toxicities.
Drug Interactions : HIV and lenti-virus used to make KYMRIAH have limited, short spans of the identical genetic material (RNA). consequently, some commercial HIV nucleic acid tests (NATs) may yield false positive results in the patients who have received KYMRIAH.
For further readings: https://en.wikipedia.org/wiki/Chimeric_antigen_receptor_T_cell https://www.novartis.com/news/media-library/science-behind-car-t-cell-ther https://www.novartis.com/news/media-library/manufacturing-car-t-cell-therapies-novartis-approach https://www.novartis.com/news/media-library/car-t-cell-therapy-infographic