Table of Contents
“According to the FDA, Clinical investigation means any experiment in which a drug is administered or dispensed to, or used involving, one or more human subjects.
For the purposes of this part, an experiment is any use of a drug except for the use of a marketed drug in the course of medical practice.
Clinical trial is the branch of health care science that determines the safety and efficiency (effectiveness) of medications, devices, diagnostic products and treatment regimens intended for human use.
Each clinical trial is designed in order to learn about potential treatment and its effect on humans.
They are clinical trials in which people volunteer to study experimental treatments, methods or experiments as a way of avoiding, identifying, treating or controlling different illnesses or disorders.
In the United States, Clinical trials are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits.
The global demand for Contract Research Organization (CRO) is estimated at USD 34500 million in 2018 and is projected to hit USD 55300 million by the end of 2024, rising at a CAGR of 8.2% between 2019 and 2024.
There are 2 main types of clinical studies:
(a) On the basis of researchers behave:
- Interventional study (experimental studies)
- Observational study
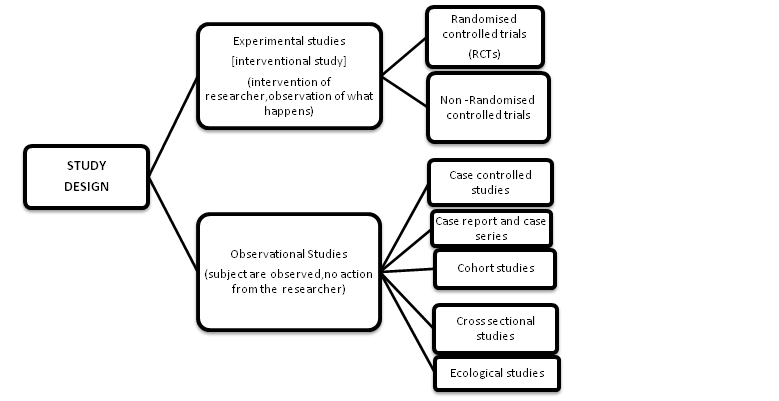
Interventional clinical study:
Interventional clinical study is performed with the purpose of studying or representing clinical or pharmacological properties of drugs/devices, their side effects and to establish their efficacy or safety.
They also include studies in which surgical, physical or psychotherapeutic procedures are inspected.
Interventional studies are often prospective and are specially tailored to evaluate direct impacts of treatment or defensive measures on disease.
Each study design has precise outcome measures that rely on the type and quality of the data utilized.
Moreover, each study design has potential limitations that are more severe and need to be addressed in the design phase of the study.
Non-randomized trial
Non-randomized trials are interventional study designs that compare a group where an intervention was performed with the group where there was no intervention.
These are suitable study designs that are most frequently performed prospectively and can suggest possible relationships between the intervention and the outcome.
On the other hand, these study designs are often subject to many types of bias and error and are not measured as a strong study design.
The randomised controlled trial
The randomized controlled trial is considered the gold standard in clinical science because it is the only proven means of preventing bias and confusing preconceptions.
Such experiments take a homogeneous sample of subjects from the study and split them into two different groups at random.
If the randomization is efficient then in all ways these two classes will be the same, all measurable confounders as well as unmeasured variables.
The experiment is then carried out in one group and not the other, and measurements of the efficacy of the experiment between the two groups are examined.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4083571/
Observational Clinical study
Observational Clinical study involves giving the participant a particular treatment in accordance with clinical practice.
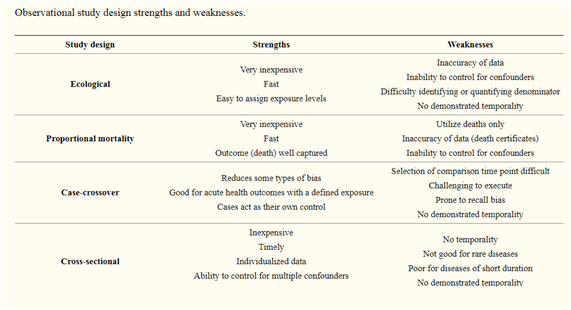
Observational studies are typically categorized into various categories such as case report or case series, ecologic, cross-sectional (prevalence study), case-control and cohort studies.
Other variants of these observational studies are also possible such as nested case-control study, case-cohort study etc.
1. Case reports and case series:
A definition of a particular or series of instances, respectively, where the medical modalities, therapeutic protocols, prognosis, etc. relevant to such characteristics are typically addressed without taking into account any protocol.
They are experimental inquiries or investigations of a patient or a group of patients in a natural, real-world clinical setting.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5630458/
2. Cross-sectional studies
Cross-sectional studies where the data concerning both exposure and outcome are collected concurrently from a selected group of the individuals in an exact population at a given point of time.
In contrast to case–control studies (participants selected based on the outcome status) or cohort studies (participants selected based on the exposure status), the participants in a cross-sectional study are just selected based on the inclusion and exclusion criteria set for the study.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4885177/
3. Ecological studies:
Group comparisons are made in these type of studies as opposed to the individual-level comparisons. Therapeutic factor and outcomes are measured at the population level.
Ecological studies may be either descriptive, such as exploring differences within populations, or considering associations.
Ecological experiments are also an interesting resource in health services science, where healthcare institutions rather than individual patients are often the object of the investigation.
For example, a present study looking at the features of general practices associated with lower coronary heart disease mortality was more alarmed with the practice at an organisational level than with individuals.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3905433/
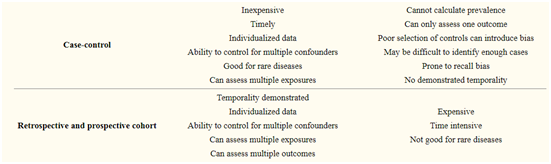
4. Cohort studies
Cohort studies are the group of subjects that represent the population of interest having certain common characteristics and studied over a sufficient period of time, for example, a cohort of survivors of Bhopal gas tragedy, the cohort of children born in a particular year.
They are selected based on the exposure status of the individual.
They are then followed over time in order to evaluate for the occurrence of the outcome of interest. Some examples of cohort studies are
(1) Framingham Cohort study,
(2) Swiss HIV Cohort Study, and
(3) The Danish Cohort study of psoriasis and depression.
These studies may be prospective, retrospective, or a combination of both of these types.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4763690/
5. Case-control studies
Case-control studies start when the outcome/disease has already occurred. Here two groups namely cases (those having the outcome of interest, i.e., a particular disease, a complication) and controls (those not having the outcome of interest) are selected and information about their exposure(s)/ risk factor(s) under consideration are collected from existing records, patient examinations, personal interviews, etc., for comparison.
In theory, the case-control study can be described simply.
First, categorize the cases (a group known to have the outcome) and the controls (a group known to be free of the outcome).
Then, look back in time to study which subjects in each group had the exposure(s), comparing the frequency of the exposure in the case group to the control group.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1706071/
6. Nested case-control study: Variation of a case-control design
Cases of a disease that occurs in a given population are reported in the nested case-control study and, for each, a defined number of matched controls is selected from among those in the group that have not attained the disease by the time the disease occurs in the event.
The type of nested case-control study depends on how the controls are selected.
i) At the end of the cohort study, controls are selected at random bases from the disease-free subjects.
Such design is known as exclusive design or total sampling of events, and is usually calculated for such design in case-control studies and odds ratio.
ii) All controls are randomly selected from the cohort at the start of the follow-up.
Here, a control at the start of study may become case during the subsequent follow-up, hence, it is called inclusive design and as the controls are representative of full cohorts, it is also known as a case-cohort study.
iii) The cohort is followed at standard intervals and as a new case is diagnosed, a control is selected from the population at-risk at that point of time.
The main feature of these studies are both case and control are selected from the matching source of population and reduce the chance of selection bias.
Additionally, these studies are cost efficient compared with full cohort studies, albeit decrease in sample size may reduce the statistical power.
Example of a nested case-control study:
To evaluate whether treatment with sodium valproate (exposure) was associated with reduced risk of the stroke (outcome), a nested case-control study was implemented with cases diagnosed with incident nonhemorrhagic stroke and controls matched for sex, year of birth, and study start date.
http://www.astrocyte.in/article.asp?issn=2349-0977;year=2014;volume=1;issue=2;spage=154;epage=159;aulast=Kumar
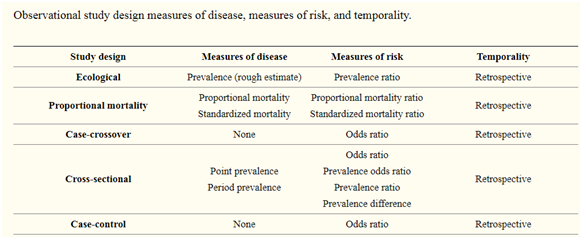
7. Measures of association:
Relative risk (RR), risk difference (RD) and odds ratio (OR) are the measures of the association repeatedly reported in observational studies. RR is the ratio of the probability of the occurrence/incidence of an event in the exposed group to that in the nonexposed group.
RD is a difference in risk between the experimental group and control group. As temporality is ascertained in cohort studies, occurrence rates and hence risks can be computed by these type of study designs. In case-control studies, the measure of association is the odds ratio.
Simply, OR is the ratio of odds of exposure among the diseased group to the odds of exposure among the non-diseased group. “
The ORs and RRs, even though conceptually different, are often mistakenly interpreted in the same way.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4083571/http://www.astrocyte.in/article.asp?issn=2349-0977;year=2014;volume=1;issue=2;spage=154;epage=159;aulast=Kumar