“Cancer biomarkers (CB) are biomolecules produced in response to the tumour by either the tumour cells or other cells within the body.
Each type of cell has its unique molecular signature and identifiable characteristics, such as levels or activities of a myriad of genes , proteins or other molecular characteristics; thus, biomarkers can facilitate the molecular definition of cancer.
We have already discuss biomarkers in detail at https://lifepronow.com/2020/08/18/what-are-biomarkers-what-are-the-types-of-biomakers/
Table of Contents
Introduction
Biomarkers are “Any measurable diagnostic indicator used to determine the risk or existence of disease” as described by the U.S.FDA or as “a function objectively assessed and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention.”
Cancer biomarkers (CB) are biomolecules formed in response to the tumour by either the tumour cells or other cells in the body, and CB may be used as a screening / early warning method for cancer, diagnosis , prognosis, or indicator of a patient’s overall outcome.
Cancer biomarkers can include:
- Proteins
- Gene mutations (changes)
- Gene rearrangements
- Extra copies of genes
- Missing genes
- Other molecules
In addition, biomarkers of cancer may classify subpopulations of patients most likely to respond to a given therapy. Biomarkers may be genes, gene products, particular cells, molecules, enzymes or hormones that can be detected in blood, urine, tissues or other body fluid.
Cancer development and mechanisms for the production of cancer biomarkers
Cancer is a multifactorial cluster of diseases that represent fundamental abnormalities involving uncontrolled cell growth and proliferation that alternate the normal cell conduct.
Molecular mechanisms exhibit alterations in the expression of multiple genes usually involve: (proto) oncogenes, tumour suppressor genes, and DNA repair genes that contribute to the development of cancer genotype and phenotype with a state of cell proliferation dysregulation events.
Cancer hallmarks hypothesis has been postulated in 2000 by Hanahan and Weinberg. Initially, they classified biological pathways for the development of cancer into six processes: proliferative signalling, avoiding growth suppression, cell death resistance (immortalization), allowing replicative immortality, angiogenesis induction and finally, invasion and metastasis activation.
Increasing evidence suggests that cancer can also be caused by epigenetic modifications such as histone modification and methylation alteration of the DNA causing changes in chromatin’s condensation state.
Genetic alterations of cancer cells, such as point mutations, gene rearrangements or amplifications, and subsequent cell division and proliferation disruptions can be expressed by releasing biomarkers of such alterations in most patients with a particular type of cancer.
Hence they can be used as biomarkers to diagnose cancer or to predict responses to different treatments.
Comprehensive understanding of the altered molecular mechanisms and cellular processes underlying carcinogenesis or cancer hallmarks may link biomarkers of cancer with their clinical usefulness in patients with cancer.
Genetic , molecular and metabolic biomarkers can be identified by applying the sequential gene mutation events that occur in cancer cells following their effects on cell proliferation and metabolism as shown in Figure below.
One of the major challenges for oncology research is to establish the definite relationship between cancer biomarkers and cancer pathology, as well as, to detect cancer in early stage beside the development of targeted therapies targeting the exact altered gene or cellular process.
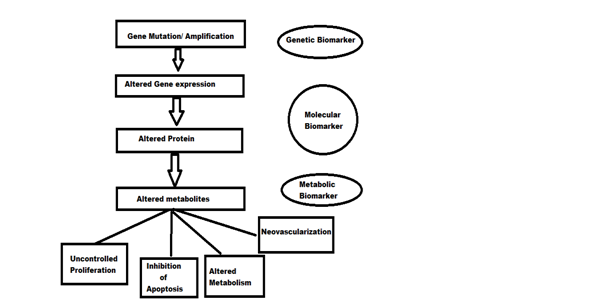
Identification of biomarkers in the process of carcinogenesis
Serum, biological fluid, and tissue Cancer Biomarkers
Understanding carcinogenic mechanisms may explain the production and release of CB in cancer cells, blood or various body fluids and thus release of these molecules and elevation during cancer initiation, growth, and progression or metastasizing.
Three mechanisms may describe mechanisms for elevating the CB levels in each of the biological fluids.
The first mechanism is the overexpression or amplification of the gene product, or the enhancement of epigenetic modifications (affect gene expression) as DNA methylation in ovarian cancer with release of such CB as protein human epidididymal secretory protein 4 (HE4).
HE4 is overexpressed in ovarian carcinoma and could be also detected in the serum.
Clinical assessment of HE4, however, revealed that it is also over-expressed in endometrial, breast, and bronchial adenocarcinoma. Typically, the second elevation mechanism could be applied to serum biomarkers, which is the cellular protein secretion or the shedding of membrane proteins.
An example of such serum biomarker is alpha-fetoprotein (AFP); an oncofetal protein with altered single peptide elevated in circulation in patients with hepatocellular carcinoma and HER2-neu, a cell membrane surface-bound tyrosine kinase, released and elevated in the serum of patients with breast cancer after proteolysis has been cleaved.
Even FDA approves HER2-neu for tracking metastatic breast cancer cases. The third mechanism is cell invasion and angiogenesis, as occurring with prostate-specific antigen (PSA). It is usually expressed by prostatic epithelium but elevation of PSA levels occurs due to distorted prostatic cell basement membrane and lymph angiogenesis.
CB’s clinical application, particularly circulating protein targets in cancer management, is evolving into a new era, especially with the availability of promising sensitive techniques implementing the discovery of “omics” cancer biomarkers in body fluids that can represent a novel, highly sensitive diagnostic tool for early cancer detection.
Of even much importance are hidden cancers that are not easily accessible, for example, nasopharyngeal, ovarian, and pancreatic cancers.
However, there is mandatory need for validation of such biomarkers. CB could be detected in cancerous cells or tissue of origin in solid tumors, bone marrow, and lymph node or as circulating cells.
CB may be found in the non-invasive specimens or samples of biological body fluid such as serum, ascetic fluid, pleural fluid or urine. CSF fluid is an effective candidate for brain cancer and CNS cancer.
In the meantime, urine is one of the promising frontiers for bladder cancer diagnosis or for monitoring patients.
It was further postulated that prostate cancer antigen 3 (PCA3) is another promising new molecular marker for prostate cancer diagnosis and follow-up.
Other examples of biological fluid sources for discovery or clinical application tool for CB are colorectal cancer stools, nipple aspirate fluid, ductal lavage and cyst fluid for breast cancer.
Clinical applications and performance indications of Cancer Biomarkers
More than 25 years ago, the clinical usefulness of CB was limited to be an effective tool for patient’s prognosis, surveillance, and therapy monitoring.
Definition of tumor markers that have been adopted by the fifth International Conference on Human Tumor Markers held in Stockholm, Sweden, in 1988 stated that
“Biochemical tumor markers are substances developed in tumor cells and secreted into body fluids in which they can be quantitated by non-invasive analyses. Because of a correlation between marker concentration and active tumor mass, tumor markers are useful in the management of cancer patients.
Markers, which are available for most cancer cases, are additional, valuable tools in patient prognosis, surveillance, and therapy monitoring, whereas they are presently not applicable for screening. Sero-diagnostic measurements of markers should emphasize relative trends instead of absolute values and cut-off levels.”
However, CB have been reported to be used also for screening of general population or risk groups, for differential diagnosis, and for clinical staging or stratification of cancer patients.
CB is often used to estimate the tumour burden and to replace a clinical endpoint and/or determine clinical benefit, harm or lack of benefit or damage.
PSA, AFP, CA125, and CEA are amongst the widely used biomarkers in clinical practise. PSA is one of the serum biomarkers commonly widely used to determine the risk of underlying prostate cancer in primary care.
Cancer antigen 125 (CA-125) can be a biomarker of the risk of ovarian cancer or a malignancy indicator but it has low sensitivity and specificity. CEA is another biomarker that is elevated in colorectal, breast , lung or pancreatic cancer patients.
A major challenge is to build promising CB for cancer patient stratification not only to predict outcome or response for therapy, providing customised treatment, but also to personalised cancer patient therapeutic strategies. Survivin and HER2-neu are among promising biomarkers in that field.
Sensitivity and specificity for evaluation of accuracy of CB
CB can be found in all of the body fluids, secretions, or tumour tissue and cells as it is released from tumour cells, or body cells in response to the tumour. CB can be detected in serum , plasma, or whole blood, including in total excretions such as urine, sputum, or CSF.
Therefore, CB may be tested both in a non-invasive and serial manner. Evaluating cancer biomarkers in tissue or cells involves biopsy of the tissue or more invasive technique than serum biomarkers.
Special techniques can detect CB in tissues but in an invasive manner than biomarkers in the serum or urine. Genetic biomarkers may be detected in tumour tissue, whole blood, or buccal mucous cells derived from DNA.
Evaluation of the diagnostic value of any test or marker is usually performed with reference to the sensitivity and specificity terms of that marker. Specificity means the marker’s ability to detect non-diseased subjects while sensitivity refers to the test’s ability to distinguish diseased subjects (patients).
Ideal biomarker
Measuring a biomarker’s sensitivity and specificity at a number of cutoff values may have a major impact on CB evaluation as we can choose a definitive cutoff value that achieves the highest sensitivity and specificity.
Increasing the cutoff point would definitely lead to an increase in test specificity or false negative patients, yet on the other hand it will decrease the number of false positives; this indicates a highly specific yet low sensitive biomarker.
Similarly, if the cutoff point is low indicating a highly sensitive yet low-specific biomarker, because there are less false negatives but more false positives.
Indeed, the biomarker ‘s accuracy and its ability to differentiate between healthy (normal) and diseased can be represented by pairs of sensitivities and specificities.
We may define the threshold or cutoff value to a diagnostic sensitivity of 100 percent or less but consider the corresponding threshold specificity. The decision threshold has to be established for use in patient care but not for accuracy assessment.
Indeed, performance assessment at the definitive point can be misleading, or this may lead to bias in comparing tests.
Ideal biomarkers must be able to distinguish specifically between cancerous and benign cases, aggressive tumours from insignificant ones; they should be of high specificity and sensitivity.
Additionally, it should be a noninvasive and inexpensive. An ideal biomarker’s characteristic features are variable and somewhat relay to the application and classification of CB. CB mostly has to fulfil the basic general properties in order to be considered ideal.
Obviously no biomarker could meet all of these requirements together, but these criteria should be highly considered for diagnostic biomarker selection:
- High clinical sensitivity: Produced by all the patients with that specific cancer (100% TPR).
- High clinical specificity: low deceptive negative (100% True negative) rate.
- Specific organ or tissue;
- Tumor burden or volume proportional: quantitatively proportional to tumour volume or progression of the disease.
- Short half-life: quick reflection of any early changes in the tumour burden to ensure proper therapy monitoring.
- Present (if any) in the serum of healthy individuals and those with benign disease at low levels;
- Metastasis which is highly discriminatory.
- Available in quantitative , standardised, reproducible and validated assessments.
- Coasting method which is inexpensive or low.
- Achieved non-invasively: detected in serum, body fluid, or easily accessible tissue.
Uses, clinical utility, and limitations of CB
Conventionally used tumour markers or CB may be proteins or glycoproteins, not likely to be involved in cancer carcinogenesis or cancer process development, but are likely to be malignant transformation by-products.
Low molecular weight , small molecules or nucleic acid markers (such as gene mutations or polymorphisms and quantitative gene expression analysis, peptides, proteins, lipid metabolites, and other small molecules are promising and are recently evaluated as potential clinically useful tumour markers, gene expression patterns and genetic alterations and defects can form part of the CB molecular classification system.
There are several CB classifications based on various aspects related to their chemical nature, mechanisms proposed for their release and applications. Six years ago, Mishra and Verma suggested a novel classification, with a focus on CB ‘s clinical utility.
They listed CB as biomarkers of prediction as biomolecules of DNA, biomarkers of detection as RNA molecules, biomarkers of diagnosis as biomarkers of proteins and biomarkers of prognosis as glyco-biomarkers.
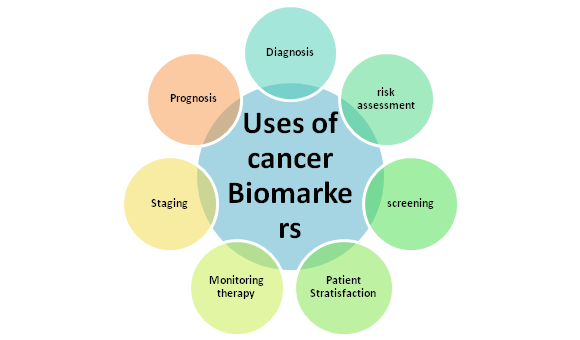
As shown in Figure below, clinical applications and uses of CB include screening and early detection, diagnostic confirmation, therapeutic response prognosis and prediction, and disease monitoring and recurrence.
Another application of CB involves indicators for cancer susceptibility and risk assessment that involve identifying individuals at high risk of developing cancer or candidates for screening and early preventive studies.
Markers for risk or susceptibility assessment include inflammation markers, oxidative stress and single nucleotide polymorphisms (SNPs), and mutations in some genes. Table 1 illustrates most of traditional, the FDA approved, and clinically relevant CB with their uses in various cancer types.
| Cancer biomarker | Organ specificity/cancer type | Application/uses |
| Prostate-specific antigen (PSA) | Prostate/BPH | Screening, diagnosis and monitoring |
| Carbohydrate antigen 125 (CA125) | Ovarian | Diagnosis, prognosis, detecting recurrence and monitoring therapy |
| Carcinoembryonic antigen (CEA) | Colorectal/hepatic | Monitoring therapy |
| Prognosis | ||
| Detecting recurrence | ||
| Screening for hepatic metastases | ||
| Carbohydrate antigen 15.3 (CA 15-3) | Breast | Monitoring therapy |
| Estrogen, progesterone receptors (ER and PgR) | Breast | Stratification/select patients for endocrine therapy |
| HER2 | Breast | Monitoring trastuzumab therapy |
| Carbohydrate antigen 27.29 (CA27.29) | Breast | Monitoring |
| Human chorionic gonadotropin-β (HCG-β) | Testicular | Diagnosis |
| Staging | ||
| Detecting recurrence | ||
| Monitoring therapy | ||
| Alfa-fetoprotein | Hepatocellular carcinoma | Diagnosis |
| Detecting recurrence | ||
| Monitoring therapy | ||
| Calcitonin | Medullary carcinoma of thyroid | Diagnosis and monitoring therapy |
| Thyroglobulin | Thyroid | Monitoring |
| CA 19-9 | Pancreatic | Monitoring therapy |
| Nuclear matrix protein 22 (NMP-22) | Bladder | Screening, monitoring and prognosis |
| Prostate cancer antigen 3 (PCA3) | Prostate | Prognostic |
Table 1. Current cancer biomarkers and uses in clinical practice.
Applications of CB in most common cancers
Cancer is a major health issue worldwide, cancer has been listed as one of the leading causes of death for both males and females over the years; an estimated 8.2 million deaths of cancer patients occurred worldwide in 2012. Every year over 11 million patients are diagnosed with cancer, and by 2020 16 million new cases are expected annually.
According to the International Cancer Research Agency (IARC)’s latest study, the GLOBOCAN worldwide estimates of cancer incidence and mortality reported in recent years and the most common types of cancers among males were lung, prostate, colorectal, liver, and urinary bladder. In the meantime, breast cancer, lung , liver, ovarian cancers were among the most common female cancers worldwide.
For many years ago, few CB have been used as an effective tool in clinical practice, while also promising CB were extensively studied for their clinical utility.
Breast cancer
Breast cancer is the most common malignancy among females and the first leading cause of cancer mortality worldwide; its prevalence is surprisingly increasing at a fast rate lately.
Hence the use of all available tools for early diagnosis and proper case management is critical. Clinically, symptoms are mostly breast lump, discharge from the nipple or changes in the skin or nipple.
The American Cancer Society’s screening recommendations suggest that women over 40 undergo mammography and a clinical breast examination annually or every other year
Diagnosis relies primarily on pathological examination; however, the role of CB in breast cancer is primarily helpful for prognosis, therapy monitoring, and follow-up. Notably, CB shows limited value for early diagnosis.
European Society of Medical Oncology has suggested assessment of ER and progesterone receptors (PR) in tissue for newly diagnosed breast cancers to assess response to hormone therapy in early and advanced breast cancer cases.
HER-2 is another prognostic marker which is most useful for selecting patients with either early or metastatic breast cancer for the treatment of Trastuzumab (Herceptin) or for predicting early breast cancer resistance to tamoxifen therapy.
Determining risk groups for breast cancer development, which must be included in the screening programme, includes the identification of genetic mutation of BRCA 1 or BRCA 2 genes, which accounts for up to 5 percent of cases of breast cancer.
Due to their high susceptibility to breast and ovarian cancer, it is powerfully recommended that women carrying BRCA1 or BRCA2 mutations undergo routine cancer screening.
It was reported that low levels of the urokinase plasminogen activator (uPA) and plasminogen activator inhibitor-1 (PAI-1) correlate with a reduced risk of recurrence of the breast cancer and shown to be strong independent prognostic factors of the newly diagnosed lymph node-negative breast cancers.
Serum biomarkers are primarily applicable as monitoring markers during therapy or to a lesser extent as prognostic markers and are typically assisted in post-operative surveillance, and CB includes CA15.3, CEA, and BR 27-29 in that category.
In advanced cases of breast cancer they are used in combination with other radiological and clinical evaluation instruments to monitor chemotherapy. Elevated serum levels of these markers can indicate recurrence or disease progression.
Prostate cancer
Prostate cancer (PCa) is one of the most common cancer in men and most common causes of male cancer-related deaths.
Strong evidence suggests that the PSA test revolutionised the screening and diagnostic landscape for prostate cancer, and the introduction of PSA as a screening test led to a sharp increase in the incidence of prostate cancer due to a shift in diagnosis at earlier stages, thereby reducing prostate cancer mortality.
Subsequently, many studies showed improvement in the sensitivity of PSA as a diagnostic marker using PSA subtractions and isoforms [−2] (proPSA) and its percentage derivative percentage of proPSA (percentage of PSA) as this fraction can help to discriminate between benign and malignant prostatic tumours in patients with PSA values from 4 to 10 μg / L.
Other novel and promising biomarkers are human kallikrein type 2, prostate cancer antigen 3 (PSA 3), and prostate stem cell antigen (PSCA) under investigation. PCA3 urine assay has a promising role to play in improving the accuracy of prostate cancer diagnoses.
Diagnosis of prostate cancer has been associated with elevated levels of metalloproteinase 2 and 9 (MMP-2 and MMP-9) members of the protease family. MMPs have been studied as therapeutic monitoring biomarkers for prostate cancer.
Ovarian cancer
Most of the patients with epithelial ovarian cancer are diagnosed late and they have clinically advanced stage III and IV on diagnosis; as a result, ovarian cancer needs a sensitive and specific diagnostic biomarkers.
One of the most widely used and conventionally used CBs is CA 125. It is recommended as a screening biomarker for women who have positive family history or are at high risk for ovarian cancer development, as well as CA125 has been used as a well-established, diagnostic biomarker in conjugation with vaginal ultrasound.
CA125 is often used as a biomarker for monitoring, decreasing after starting chemotherapy or surgery, which correlates with a favourable basal response level of CA125, two weeks before starting any therapeutic intervention, follow-ups and continuing monitoring of its level at regular intervals are strongly recommended.
Other biomarkers have been studied extensively in ovarian cancer monitoring and prognosis prediction but further studies are required to better validate their exact function.
This panel includes kallikreins (5–9), osteopontin, Her-2 / new, inhibitor-associated tumour, CEA, trypsin inhibitor, hCG, interleukin-6 ( IL-6), prostasin, TPA, lysophosphatidic acid, plasminogen activator inhibitor-1 (PAI-1).
Colorectal cancer
CRC ranks 3rd among all cancers worldwide. An estimated one million new cases are diagnosed and half a million deaths are registered annually. The rectum with 38 percent of all cases is the most popular location for colorectal carcinoma followed by sigmoid accounting for 29 percent of cases.
As advised, the CRC screening programme should be aimed at all asymptomatic individuals over 50 years old. The National Academy of Clinical Biochemistry (NACB) recommends that all subjects 50 or older undergo colorectal cancer screening. There are several screening methods.
The Fecal occult blood test (FOBT) is the most widely used CB in stoles. Checking for blood in stools involves either detecting blood globin fraction (haemoglobin) by faecal immunochemical examination, or the guaiac test that tests the pseudo-peroxidase role of the haemoglobin fraction. Characterized CEA in 1965, it was implemented into clinical practise.
It is commonly used as a universal or non-organ, tissue-specific marker for tumours. Because of its low sensitivity and specificity, CEA is not used in CRC screening, apart from the low prevalence of CRC in asymptomatic populations; however, it is a very effective biomarker for prognostic and therapy monitoring.
At the beginning of therapy CEA estimation is recommended then every 1–3 months during the therapeutic regimen, it is also the marker of choice for metastatic CRC cases.
CA19-9 was used as a prognostic marker, in the monitoring of CRC after surgical resection and in advanced cases as a monitoring marker for therapeutic intervention.
Other CB under investigation are CA242 and tissue inhibitor of the metalloproteinases type 1(TIMP-1) and both may complement CEA in the surveillance of the patients with colorectal cancer.”
References:
https://www.sciencedirect.com/science/article/pii/S1574789112000117
https://www.mycancer.com/resources/what-are-biomarkers/
https://www.intechopen.com/books/role-of-biomarkers-in-medicine/cancer-biomarkers