“Case report form (CRF) is designed to collect the patient data in a clinical trial as per the clinical trial protocol”
Table of Contents
What is a case report form
In plain English, we can say that CRFs are the “forms” where patients related data is entered. These forms are built as per the requirement given in clinical trial protocol.
ICH defines it as “A printed, optical or electronic document designed to record all of the protocol – required information to be reported to the sponsor on each trial subject.”
Site personnel such as clinical research coordinator and principal investigator capture the subject’s data on the CRF, which is collected during their participation in a clinical trial/study.
CRF must be designed for optimal collection of data in accordance with the clinical trial protocol, regulatory requirements and shall enable the clinical research to answer the trial related questions.
A well-designed CRF represents the essential contents of the study protocol.
The case report form is designed once is protocol is finalised for the study and it can hold modified if a protocol is an amended.
Understand the various terms used
In this example, there are two forms Demography and Body temperature CRF, which are under one folder which is named as screening
The questions like date, result, gender is called variables and space where a site can enter the data are called “fields.
So now we have learnt below terminology regarding case report form.
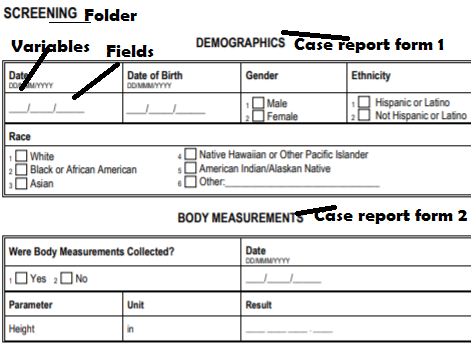
- Folder (screening)
- Forms (or case report form)-Demography and Body measurement
- Variables-All the questions like i.e Date, Date of birth etc
- Fields-Places adjacent to variables where information/results are entered
You can find many examples of case report form on below link
Type of case report form
There are two types of case report form-Paper and electronic case report forms
Paper CRF is the traditional method of data collection and a reasonable choice if studies are small or vary in design, while electronic CRFs are considered if studies are big with similar designs.
Nowadays, the electronic case report form is preferred over paper case report form
Advantages of electronic case report form (eCRF)
- They are less time consuming
- It allows sponsor/pharmaceutical companies to carry out large multicentric studies at the same time due to the ease of administration.
- Data entry is easy and errors can be minimised
- Duplication of CRF pages is avoided as repetitive data such as protocol ID, site code, subject ID will be generated by the system automatically from the first page to all others Case report forms.
- In eCRF it is simple and fast to connect the data between two related CRF.
- Rapid query resolution minimizes the time taken on obtaining site/investigator clarification and thus clean data is collected very easily, resulting in quicker locking of the database, faster regulatory submission and eventual approval.
- Designing a paper CRF is a tedious job which may lead to data errors and misconclusions,
- Chances of error during data transfer from the source document to paper CRF are quite common.
Disadvantages of eCase report form
an electronic case report form is designed on a tool that is called eDC tool.
Installation of the tool is complex and quite costly. maintenance of the software and high investment cost.


