Chimeric antigen receptors combine numerous facets of normal T cell activation into a single protein.
They link an extracellular antigen recognition domain to an intracellular signalling domain, which can activate the T cell when an antigen is bound.
Chimeric antigen receptors (CARs) are self-possessed of four regions:
- Antigen recognition domain
- Extracellular hinge region
- Transmembrane domain, and
- Intracellular T-cell signaling domain.
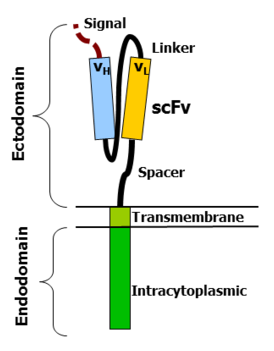
Antigen recognition domain: The antigen recognition domain is showing outside the cell, in the ectodomain portion of the receptor. It interacts with the potential target molecules and is responsible for targeting the CAR-T cell to any cell expressing a matching molecule.
The antigen recognition domain is typically derived from the variable regions of a monoclonal antibody linked collectively as a single-chain variable fragment (scFv). An scFv is the chimeric protein made up of the light (VL) and heavy (VH) chains of the immunoglobins, connected with a short linker peptide.
These VL and VH regions are selected in advance for their binding capability to the target antigen (such as CD19). The linker between the two chains consists of the hydrophilic residues with elongated glycine and serine in it for flexibility as well as stretches of glutamate and lysine for added solubility.
In addition to the scFvs, non‐antibody‐based approaches have also been used in order to direct CAR specificity, usually taking advantage of ligand/receptor pairs that normally bind to each other. Cytokines, instinctive immune receptors, TNF receptors, growth factors, and structural proteins have all been successfully used as the CAR antigen recognition domains.
Hinge region(Spacer) :The hinge, also known as spacer, is a small structural domain that sits between the antigen recognition region and the cell’s outer membrane. An ideal hinge enhances the elasticity of the scFv receptor head, reducing the spatial constraints between CAR and its target antigen.
This promotes the antigen binding and synapse formation between the CAR-T cells and target cells. Hinge sequences are often based on the membrane-proximal regions from other immune molecules that includes IgG, CD8, and CD28.
Transmembrane domain:The transmembrane domain is a structural constituent, consist of a hydrophobic alpha helix that spans the cell membrane.
It anchors the CAR to the plasma membrane, bridging the extracellular hinge and also antigen recognition domains with the intracellular signaling region.This domain is necessary for the stability of the receptor as a whole.
In general, the transmembrane domain from the most membrane-proximal constituent of the endodomain is used, but different transmembrane domains effect in different receptor stability.
The CD28 transmembrane domain is known in order to result in a highly expressed, stable receptor.Use of CD3-zeta transmembrane domain is not recommended, as it can result in incorporation of the artificial TCR into the native TCR.
Intracellular T-cell signaling domain: The intracellular T-cell signaling domain lies in the receptor’s endodomain, within the cell.After an antigen is bound to the exterior antigen recognition domain, CAR receptors group together and transmit an activation signal.
Then the internal cytoplasmic end of the receptor continues signaling inside the T cell. Normal T cell activation relies on the phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) present in the cytoplasmic domain of CD3-zeta.
In order to mimic this process, CD3-zeta’s cytoplasmic domain is commonly used as the main CAR endodomain component. Other ITAM-containing domains have also been tried, but are not as efficient.
T cells also require co-stimulatory molecules in addition to CD3 signaling to persist after activation. For this cause, the endodomains of the CAR receptors normally include one or more chimeric domains from co-stimulatory proteins.
Signaling domains from a wide range of co-stimulatory molecules have been successfully tested and include CD28, CD27, CD134 (OX40), and CD137 (4‐1BB).
https://www.creative-biolabs.com/car-t/tcr-design-construction.htm https://en.wikipedia.org/wiki/Chimeric_antigen_receptor_T_cell